Quick Links
Meet
the Commercial Team
Super User
Senior Territory Manager- Cattle, Colby Flatt
Bimeda is pleased to announce the appointment of Colby Flatt as Senior Territory Manager for the region of Southern Missouri, Eastern Oklahoma and Arkansas. Colby will be an integral part of the US Cattle Sales team. Colby brings years of experience in the Animal Health industry in both manufacturing and distribution. Colby and his wife, Tiffany reside in Cherryvale, Kansas and have two boys. Colby is actively involved in the ranching industry with his family, is a part time team roper and enjoys boating with the family.
Bimeda is proud to add Colby Flatt to the US Cattle Sales Team. Please feel free to reach out to Colby at or 620-870-9100 and welcome him to the team.

Colby Flatt
Welcome Casey Story, Senior Territory Manager – Cattle, Southeast US
Bimeda is pleased to announce the appointment of Casey Story as Senior Territory Manager. Casey will be an integral part of US Cattle Sales team in the states of Kentucky, Tennessee, Mississippi, Alabama, South Carolina, Virginia, West Virginia and Georgia. In this role, he will be responsible for the sale of Bimeda Cattle products into his territory.
Casey brings years of solid animal health industry experience in this area from former roles with Purina, Neogen and Central Life Sciences. He also brings a lifetime of practical experience working on his family's multi-generational cattle operation in Kentucky. He is a graduate of the University of Kentucky.
Casey and his family reside on his family's farm in Flemingsburg, KY where he continues to be dynamically involved. He is also an active member in many state and national cattlemen's associations.
Bimeda is proud to add Casey Story to the US Cattle Sales Team. Please feel free to reach out to Casey at or 606.209.5566 and welcome him to the team.
Bimeda Welcomes New National Sales Director-Cattle
Effective immediately, Ron is serving a newly created role of National Sales Director-Cattle as Bimeda expands their US Cattle Sales footprint.
Ron grew up in animal agriculture and has been a part of it throughout his life. Growing up part of his life on the Four Sixes Ranch and continuing that tradition with management of various ranches like, Pitchfork Land and Cattle Company and Smith Ranches. These experiences have given Ron a very unique understanding of our cattle customers. He also spent a great deal of time in supporting industries including animal health through his work at Boehringer-Ingelheim as a Territory Manager. Most recently, Ron helped lead the industry while working for Global Vet Link and Radica. There his roles included helping life science companies manage data intelligence and digital compliance needs directly with his customers and through his role of National Sales Manager.
In his role at Bimeda, Ron will manage our Cattle sales team structure, development, and path to progression with future expansion. He will have the 2024 sales team of 11 reporting to him and will report directly to me in this role.
Ron lives in the Amarillo Texas area (Groom, TX) where he enjoys equine activities centered around roping and reigning, as well as helping his father manage their cow/calf ranch locally.
Jim Lovin, Strategic Accounts Manager-Swine
Bimeda is pleased to announce the appointment of Jim Lovin as a Strategic Accounts Manager - Swine. Jim will focus on the development and management of both core Bimeda products and custom vaccine line for the swine industry.
Jim joins Bimeda with many years of Animal Health experience along with a passion for and experience in the swine industry. Jim started his career with Upjohn Animal Health, spent 13 years with Bayer Animal Health and most recently was a National Accounts Swine Manager for Furst McNess Company.
Jim lives in Ames, Iowa and can be reached at .
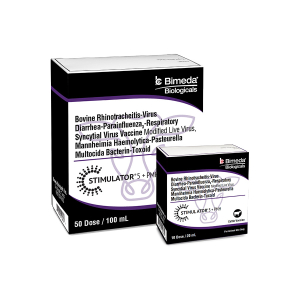
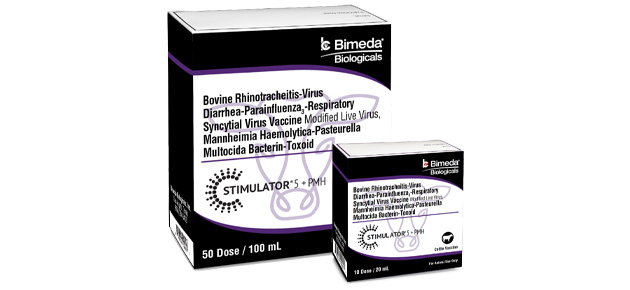
Bimeda Biologicals® Launches Stimulator® 5 + PMH
Bimeda Biologicals® is pleased to announce the newest addition to their Stimulator® line of modified live virus (MLV) vaccines: Stimulator® 5 + PMH. With this launch, Bimeda Biologicals adds to their broad portfolio of cattle vaccines another effective combination vaccine that helps protect cattle against some of the most common—and costly—viral and bacterial pathogens associated with Bovine Respiratory Disease (BRD).
This vaccine takes Bimeda Biologicals 5-way viral vaccine—that has proven its ability to go toe-to-toe with some of the most commonly used and more recognized MLV vaccine brands in large-scale research trials—and combines it with bacterial antigen to provide veterinarians and cattle producers a new, cost-effective option to help keep the cattle that they care for healthy.
Stimulator 5 + PMH has been shown to be effective for the vaccination of healthy cattle five months of age or older against Infectious Bovine Rhinotracheitis (IBR), Bovine Viral Diarrhea Type 1a (BVD I), Bovine Viral Diarrhea Type 2 (BVD II), Parainfluenza3 (PI3), Bovine Respiratory Syncytial Virus (BRSV) as well as Mannheimia haemolytica and Pasteurella multocida.
This product, like other combination vaccines, requires the user to aseptically rehydrate the freeze-dried modified live vaccine with the accompanying bottle of bacterin-toxoid. Mix reconstituted vial well. Administer 2.0 mL subcutaneously in the side of the neck followed by a second dose of Mannheimia haemolytica–Pasteurella multocida (PMH BAC™) to be given 14 to 28 days after the first dose.
For more information about the new products, visit our Products page.
U.S. Product Portfolio
Custom Vaccines
BETTER PREVENT THE PREVENTABLE
BY CHECKING ALL THE BOXES WITH A CUSTOM-MADE VACCINE
It’s hard to “check all the boxes” if you are designing a vaccination protocol that covers all the disease-causing bugs your animals may encounter, especially if you are limited to using only commercial vaccines. Read
A custom vaccine, also known as autogenous vaccine, is a useful tool veterinarians and livestock producers can use to address new or uncommon strains of viral and/or bacterial pathogens affecting their animals when commercial vaccines just aren’t getting the job done.
Custom vaccines are killed (inactivated) viral and/or bacterial vaccines that contain herd-specific antigens isolated and identified through diagnostics. Like most commercial killed vaccines, autogenous vaccines also include an adjuvant to improve the vaccinate's immune response.
Our experienced staff has been producing custom vaccines for veterinarians and the producers they service for 20+ years and our entire team strives day-in and day-out to provide you with unparalleled customer service from start to finish.
The USDA approves the use for custom (autogenous) vaccines to protect animals against disease-causing organisms, by or under the direction of a veterinarian, when:
- A fully licensed commercial vaccine is not available
- The organism exhibits a high degree of genetic variation and requires improved antigen specificity
- When commercially licensed products have failed to provide adequate protection
Custom Vaccines By Species
Custom Cattle Vaccines
Common Custom Vaccine Pathogens:
- Moraxella bovis and Moraxella bovoucli (pinkeye)
- Unique serovars of Salmonella
- Unique isolates of Mycoplasma bovis
- Unique serotypes of M. haemolytica
- BVD Type 1b
- Bibersteinia trehalosi
Resources
Custom Swine Vaccines
Focus Custom Vaccine Pathogens:
- Clostridium perfringens
- Clostridioides difficile
- Escherichia coli
- Mycoplasma hyorhinis
- M. hyopneumoniae
- M. hyosynoviae
- Glaesserella parasuis
- Streptococcus suis
Resources
Custom Sheep & Goat Vaccines
Common Custom Vaccine Pathogens:
- Unique serotypes of M. haemolytica
- Corynebacterium pseudotuberculosis / Caseous lymphadenitis (CL)
Resources
For more information on our custom vaccines contact us
Bimeda USA
Bimeda® Biologicals
1702 North Bell Street,
San Angelo, Texas
76903
Phone: 1 800 284 8403

NO SMOKE & MIRRORS.
JUST SOLID SCIENCE.
Stimulator® vaccines were designed to stimulate a strong, protective immune response in stocker and feeder cattle against the five most common viral pathogens associated with Bovine Respiratory Disease (BRD):
- Infectious Bovine Rhinotracheitis (IBR)
- Bovine Viral Diarrhea Type 1 (BVD I)
- Bovine Viral Diarrhea Type 2 (BVD I)
- Bovine Respiratory Syncytial Virus (BRSV)
- Parainfluenza3 (PI3)
DOWNLOAD | STIMULATOR DETAILER
Why Choose Stimulator Vaccines?
![]() RELEVANT
RELEVANT
Stimulator vaccines were developed 10-20 years after most other commonly used straight MLV vaccines. As a result, an additional decade’s worth (or more) of insights and understanding were used to build Stimulator vaccines. We selected strains to maximize cross-protection against the most prevalent subtypes affecting your cattle.
![]() QUALITY
QUALITY
All viral antigens contained in Stimulator vaccines have been genetically tested to confirm stability and purity to ensure they have not mutated during the production process.
![]() SAFE
SAFE
Unlike most MLV’s for cattle, no fetal bovine serum is used at any stage of production of the viruses or the cells contained in Stimulator vaccines. This guarantees that no extraneous, unwanted bovine viruses are introduced from fetal bovine serum.
![]() EFFICACY
EFFICACY
Stimulates protective immunity so your cattle are better equipped to remain healthy and productive when faced with common viral challenges.

PROTECT YOUR CATTLE AND YOUR
BOTTOM LINE.
Our PRO-BAC™ vaccine line was developed specifically for stocker and feeder cattle and offers the most complete protection against five of the most common BRD-causing bacteria:
- Mannheimia haemolytica Type A1 (MhA1)
- Mannheimia haemolytica Type A6 (MhA6)
- Pasteurella multocida (Pm)
- Histophilus somni / Haemophilus somnus (Hs)
- Salmonella typhimurium (St)
No other vaccines available on the market can say the same.
All of the PRO-BAC vaccines are powered by our proprietary adjuvant Reveal ATS™. Learn more about the Reveal ATS Advantage
DOWNLOAD PRO-BAC - FEATURES, BENEFITS & COMPARISONS TO OTHER PRODUCTS PRO-BAC - OVERVIEW & IMPORTANCE OF PREVENTION PRO-BAC ADMINISTRATION GUIDE
Why Choose PRO‑BAC VACCINES?
![]() QUALITY BACTERIAL ANTIGENS
QUALITY BACTERIAL ANTIGENS
Our antigens are grown in specific conditions to maximize expression of all virulence factors, which optimizes post-injection antigen presentation in order to elicit a stronger immune response to better protect your cattle against the bacterial challenges they may face later on—whether that is on the ranch or in the feedlot.
![]() CONVENIENCE
CONVENIENCE
Available in both 50-dose and 100-dose bottles, PRO-BAC vaccines come ready-to-use requiring no chute-side mixing and are administered in a BQA-friendly 2.0 mL subcutaneous (SQ) dose in the side of the neck.
![]() VERSATILITY
VERSATILITY
Choose the right antigen combination to plug into your current vaccination protocol to fill the gaps other vaccines aren’t covering to set your calves up to perform on the ranch and in the feedlot.
![]() EFFICACY
EFFICACY
Our unique manufacturing process and proprietary adjuvant system (Reveal ATS™) results in vaccines that provide a high level of protection against the costliest BRD-causing bacteria without the harsh side effects often associated with the vaccination of mid- to high-risk cattle.
Learn more about the Reveal ATS Advantage
DEALING WITH HIGH PULL RATES, POOR TREATMENT RESPONSE AND SWOLLEN JOINTS IN RECENTLEY WEANED CALVES?
YOUR VACCINE PROTOCOL MAY BE MISSING A KEY PIECE.
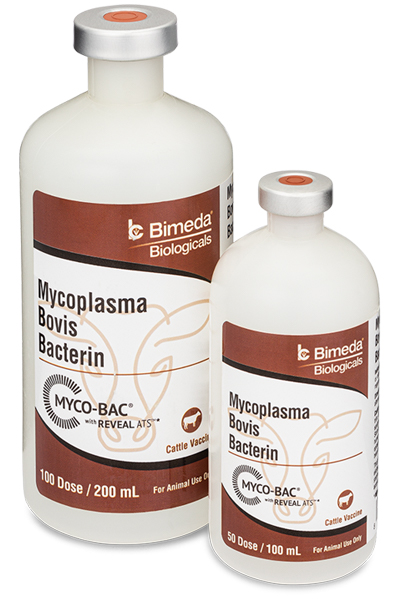
Mycoplasma bovis is one of the most prevalent of all the bacterial pathogens affecting young cattle during periods of elevated stress such as weaning and transportation. In general, the higher the “risk classification” of a particular group of cattle the greater the possibility of a Mycoplasma problem. Read
The typical “myco” infections begin to show up about 3 weeks after exposure and can cause respiratory disease as well as crippling arthritis. Due to it’s extremely small cell size and lack of a ridged cell wall, many antibiotics are rendered ineffective against M. bovis resulting in increased pull rates, poor treatment response and unusually high death rates.
MYCO-BAC® was the first fully licensed Mycoplasma bovis (also referred to as ‘Mycoplasma’ or ‘Myco’) vaccine approved by the USDA in 2002. It was originally developed by the team at Texas Vet Lab, Inc. following years of clinical observation and diagnostic/laboratory research as well as significant experience producing effective autogenous vaccines for feedlot and stocker veterinarians and consultants from across the country.

MYCO-BAC is powered by our proprietary adjuvant Reveal ATS™. Learn more about the Reveal ATS Advantage
DOWNLOAD | MYCO-BAC LABEL
The Benefits of Vaccinating with MYCO‑BAC
COST SAVINGS
When it comes to Mycoplasma bovis, prevention is almost always cheaper than treatment. Not only will a myco infection negatively impact cattle’s’ performance by keeping them off feed and water, effective treatment options are limited which often leads to high labor and drug expenses.
REDUCED MORBIDITY & MORTALITY
Field trials coupled with expansive experience and understanding of the disease support that giving MYCO-BAC, according to the labeled 3-dose regiment administered on days 0, 7 and 14, will reduce morbidity and mortality as well as increase treatment response success in vaccinated cattle.
EFFECTIVE
Our unique manufacturing process combines quality bacterial antigen with our proprietary adjuvant system (Reveal ATS™) to create a strong, specific immune response to prevent and control respiratory disease due to M. bovis.
Learn more about the Reveal ATS™ Advantage

PREVENTING THE PREVENTABLE
WITH AN EFFECTIVE COMBINATION.
An effective combination vaccine that helps protect cattle against some of the most common—and costly—viral and bacterial pathogens associated with Bovine Respiratory Disease (BRD).
Available Sizes
10 dose, 50 dose
DOWNLOAD | STIMULATOR 5 + PMH DETAILER